Quality and regulatory affairs
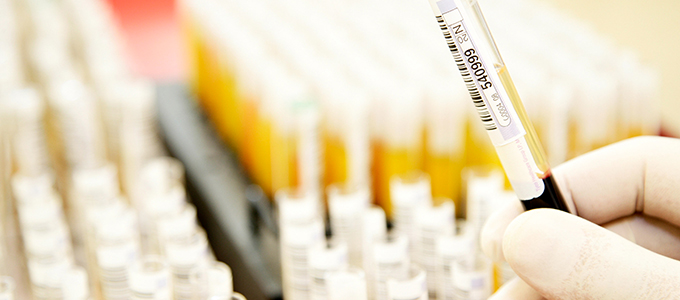
This team includes the regulatory training, compliance and certification, quality control and audit departments.
It oversees the application of the standards describing exactly how blood is to be collected and the blood components are to be transformed, analyzed, stored and transported.
This team is also responsible for investigations concerning transfused products, studies of past donations made by donors who are now known to be carriers of infections that can be transmitted through blood, and notifying donors with positive viral markers.
To learn more about Héma-Québec’s achievements, read the annual report and the scientific activities report.
Job categories
.jpg)
Find an exciting job
Discover our job categories and put your talents to use in the field that you are passionate about.
LearnHéma-Québec at a glance
In 2022—2023:
- 1,677 employees
- Thousands of volunteers
- 233,379 blood, plasma, stem cell, human tissue, and mother’s milk donors
- 773,611 products distributed (all donation types)
- 30,083 tests conducted for Québec hospitals
Thank you for your contribution to our mission!
