Individual Risk-Based Donation Qualification
The blood donation qualification questionnaires were replaced by gender-neutral questionnaires. Each person answers the same questions as a result of this change, regardless of sex, gender or sexual orientation. This change enables a more inclusive approach to people hailing from LGBTQ+ communities.
Frequently Asked Questions
Eligibility to donate blood products is now based on an individual at-risk behaviour evaluation rather than a population-based evaluation.
With this new approach, each person wishing to donate blood, plasma or platelets, regardless of sex, gender or sexual orientation, is asked if they had a new partner or multiple sexual partners in the last three months and, if so, if they had anal intercourse.
Owing to physiological differences that make the distinction between people assigned as male and female at birth, safety measures are applied during a blood donation. These measures have been implemented to prevent risks that may harm the health of those donating blood products.
As a result of implementing gender-neutral questionnaires for blood, plasma and platelet donations, anyone wishing to donate blood products answers the same questions regardless of sex or gender identity. An evaluation by an Héma-Québec medical staff member is no longer required. A fact sheet for trans and non-binary people lays out the risk for donors’ health and is available at blood drives upon request. The sheet is also available to download here.
Note: While transitioning to gender-neutral donation qualification questionnaires for all blood products, Héma-Québec has also begun working on plans to improve its registration processes by making them more inclusive for trans and non-binary individuals, always without compromising security. We are working in close collaboration with research professionals at UQAM to consult LGBTQ+ communities in order to improve the blood donation experience for trans and non-binary individuals.
A transition from a population-based approach to an individualized approach is now possible because of evidence data currently available. Donation bans are based on behaviours proven to present a higher risk of contracting a sexual infection transmitted by blood. The goal is to maintain the same high level of safety of blood products all the while being more inclusive.
As a result, more people hailing from LGBTQ+ communities are eligible for donations, for they do not present any individual at-risk behaviours.
Héma-Québec has increased its efforts to obtain evidence data and ease qualification criteria for men having sex with men since 2013 without affecting the safety of recipients.
Until now, the data collected have made it possible for Héma-Québec to submit five requests for easing qualification criteria that affected men who had sex with men. Health Canada has accepted these requests upon proving they did not affect the safety of blood products.
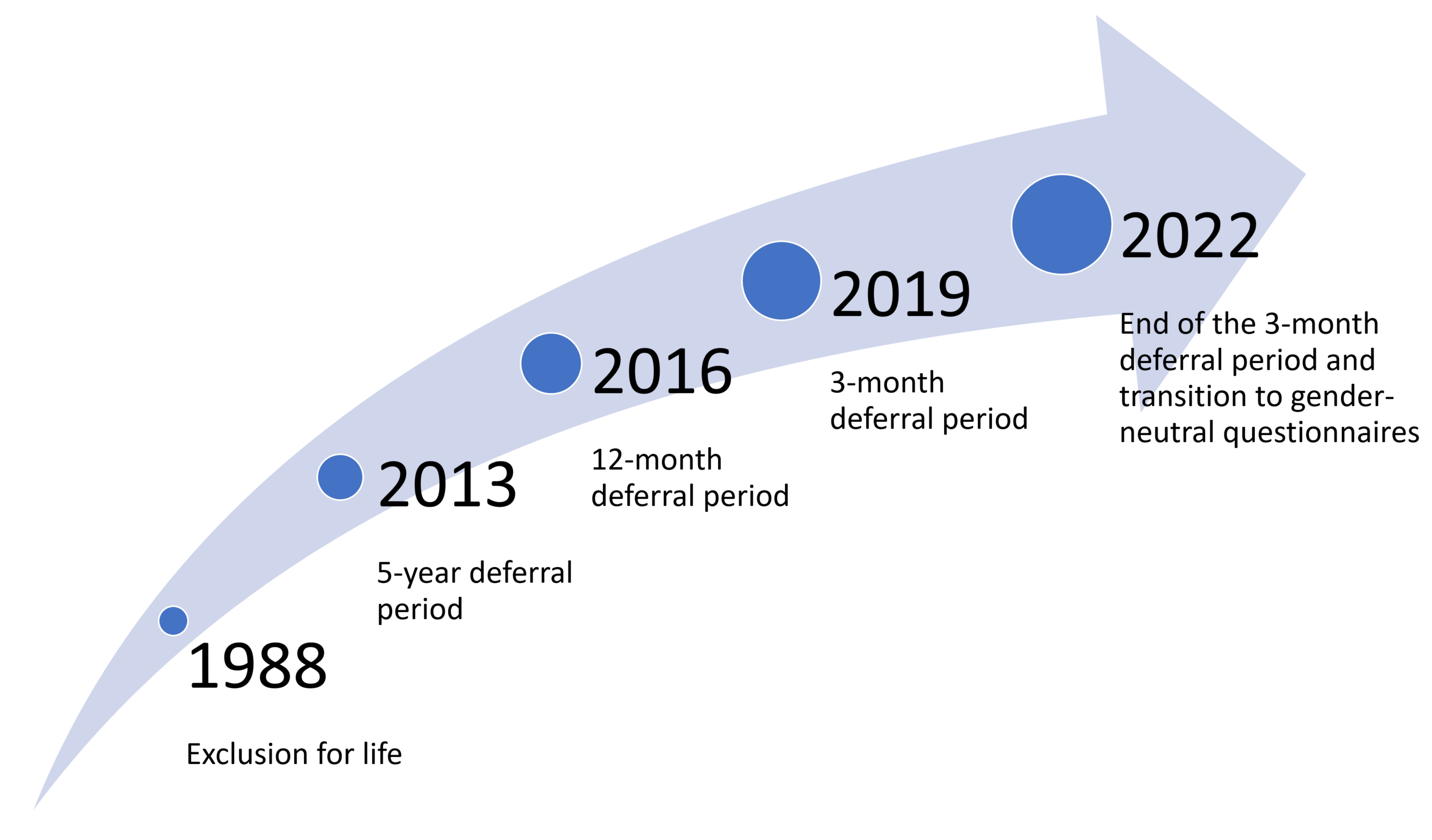
Since October 2, 2022: Donation eligibility based on an individual at-risk behaviour evaluation has been implemented (rather than a population-based evaluation). Each person who is sexually active may donate plasma if there has been no anal intercourse with a new partner or multiple partners in the last three months.
Since December 4, 2022: Like plasma donation, eligibility for blood and platelet donations is based on an individualized assessment of risk behaviors rather than on an assessment of targeted groups.
What has been done to make blood product donation more inclusive and accessible to men having sex with men as well as sexual and gender diversity communities was based on evidence data without compromising the safety of blood products.
Héma-Québec has implemented a more inclusive qualification approach and is keen to better understand and improve the donor experience provided to LGBTQ+ communities. Since 2020, it has in part been working with a research team at UQÀM to pinpoint areas of improvement and implement them gradually.
New gender-neutral questionnaire
The gender-neutral questionnaire enables an individualized approach, i.e., donors are asked the same questions, regardless of their gender or sexual orientation. This approach differs from the previous approach of a population-based risk assessment, which targeted a specific group of people.
A transition from a population-based approach to an individualized approach was now possible because of evidence data currently available. Donation bans are based on behaviours proven to present a higher risk of contracting a sexual infection transmitted by blood. The goal is to maintain the same high level of safety of blood products all the while being more inclusive.
As a result, more people hailing from LGBTQ+ communities are eligible for donations, for they do not present any individual at-risk behaviours.
The blood donation qualification questionnaires were replaced by gender-neutral questionnaires. Each person answers the same questions as a result of this change, regardless of sex, gender or sexual orientation.
Thus, there are no longer gender-specific questions. As a result, questions about pregnancy history and sexual risk behaviors are asked of everyone who comes in to donate.
In addition, we ask new questions to evaluate individual risk. In the last three months, we will ask if you had:
- a new sexual partner; or
- more than one sexual partner.
If you answer yes to one of the questions above, we will ask if you have had anal intercourse. If so, you cannot donate in the three months following the most recent anal intercourse.
If you answer no, you may donate as long as you meet the other eligibility criteria.
We realize that questions relating to specific sexual practices can be embarrassing and uncomfortable. However, these questions are crucial to evaluate individual risk and guarantee the highest level of safety of blood products for recipients.
The expression “new partner” means someone you have not had sex with before, or someone you had sex with in the past and have had sex with again.
Not necessarily if the relationship is exclusive, for the person is not a new partner. If it had been proven that the partner was not exclusive during the three-month period (for example, because of a temporary break-up), that partner would be considered a new partner.
The expression “sexual relations” refers to the following acts with or without a condom or other means of protection: vaginal penetration (contact of a penis with a vagina), oral sex (contact of the mouth or tongue with the vagina, penis or anus), and anal penetration (contact of the penis with the anus).
Anal intercourse presents a higher risk of acquiring sexually transmitted infections and blood infections. This risk is a little higher in anal intercourse than it is in oral and vaginal intercourse.
PrEP is a highly effective medical treatment used to prevent HIV transmission through sexual contact. When someone is on PrEP or PEP treatment, however, a small viral load of HIV may not be detected, possibly presenting a risk of transmission through transfusion.
Though condoms are an excellent safe-sex option, they are not always effective, for they can break or slip. Therefore, condoms are not a reliable indicator of donation safety.
Our questionnaire has been designed to reach out to the largest group of people possible to evaluate and determine the risk of new exposure to some viruses during the latent period (also called “window period”), i.e., the period between exposure to an STI and the moment when the infection can be detected by laboratory analysis.
Anyone who said they had more than one sexual partner in the last three months is included in the question “Have you had more than one sexual partner in the last three months?” and must also answer the subsidiary question regarding anal intercourse.
We are aware that some people may have sexual intercourse with a number of partners who are not new. According to current available data, people who have more than one sexual partner and have anal intercourse are more at risk of acquiring blood-transmitted infections.
*Polyamory is the practice of partners having romantic relationships with more than one person at a time. Each partner knows about the other person and accepts them.
The blood donation qualification questionnaire is gender-neutral, i.e., each person answers the same questions, regardless of sex, gender or sexual orientation. However, owing to physiological differences that make the distinction between individuals assigned as male and female at birth, safety measures are applied during a blood donation. These measures have been implemented to prevent risks that may harm the health of those donating or receiving blood products.
The person’s sex is recorded in the donor file on the basis of photo identification presented at the time of the donation. Héma-Québec recognizes that the person’s sex on the identification documents does not always correspond with the donor's gender identity. For this reason, work is underway to improve the registration process to make it fully inclusive for trans and non-binary donors while still ensuring their safety.
Product safety for recipients
For Health Canada, a department regulating transfusion systems across the country, to accept a request to change criteria selection, we need to provide Health Canada with an exhaustive bid that includes evidence data showing that changes do not compromise the safety of blood products. Progress in epidemiological knowledge and scientific proof support these changes.
Donors are asked about their sexual behaviours in the last three months, which may have increased their risk of infection. These are questions based one a one-on-one risk evaluation approach instead of a population-based evaluation, all the while maintaining the safety of blood supply.
All donations are tested to detect blood-transmitted diseases before being sent to hospitals.
Infectious agent screening tests and the blood donation qualification questionnaire are two highly effective strategies to maintain the safety of blood products.
We need to consider the latent period, i.e., the period when most recent infections cannot be detected through tests but can be transmitted through blood.
The latent period could last anywhere between a few days to a few weeks, depending on a variety of tests. Add to this an extra period for precaution, which would explain the three-month ban.
No. The risk of infection transmission is determined by the mechanism (for example, anal, vaginal, oral intercourse) and the number of people infected among the population (also called prevalence). The gendered questionnaire excluded a population with a higher prevalence, whereas the gender-neutral questionnaire will put more emphasis on the transmission mechanism, making the approach more inclusive.
It is possible that people taking part in riskier sexual behaviours among a population with a smaller prevalence were once eligible for donations, but this will no longer be the case. Even though these people posed an extremely low risk, this risk is higher than a man who had exclusive sexual intercourse with another man.
