Blood management in Québec
Donating and receiving blood has never been as safe as it is today.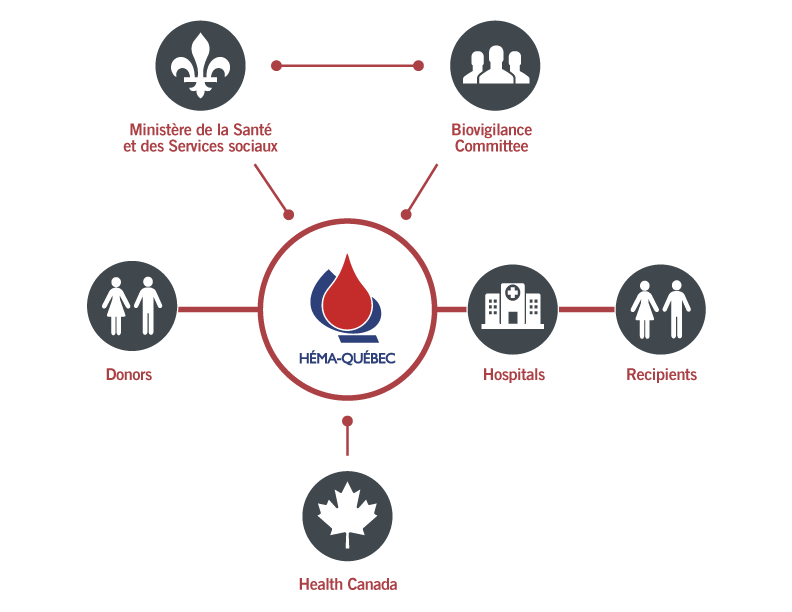
The Québec blood management system was reorganized at the end of the 1990s. It is based on a clear and precise division of responsibilities among its three major components:
Héma-Québec
In keeping with the main recommendations of the Gélineau and Krever reports, the Minister of Health and Social Services announced the creation of Héma-Québec on March 30, 1998 as part of the reorganization of Québec's blood management system.
Héma-Québec is a not-for-profit organization whose principal mandate is to supply hospitals with safe, superior-quality blood products. As the blood supplier, Héma-Québec is responsible for donor recruitment, blood collection, the quality of blood products and their distribution to hospital centres.
An organization that is not directly dependent on the government and which has its own board of directors, Héma-Québec is fully integrated and in line with Québec’s healthcare network. Our board of directors is made up of representatives from all links of the transfusion chain, from the donor to the recipient.
We comply with Health Canada’s safety standards.
We collaborate with and maintain close links to Canadian Blood Services, the agency responsible for the blood management system for the rest of Canada. An online link has been established between the donor banks of these two organizations.
Hospitals
Hospital centres are responsible for the quality and safety of blood transfusions.
Under Québec’s blood management system, hospitals assume the cost of their blood and blood product supply as well as storage and inventory management. They are also responsible for providing patients with superior-quality blood transfusions.
Transfusion medicine committees are set up in the hospitals to ensure that transfusion-related medical practices are compliant with international guidelines and standards.
Héma-Québec delivers blood components directly to about a hundred hospitals in Québec.
Biovigilance Committee
Under the authority of the Minister of Health and Social Services, the Biovigilance Committee is in charge of public health; it monitors the risks associated with blood transfusions.
The committee's views are based on the data collected from all the links in the transfusion chain (donors-suppliers-hospitals-recipients). This data is subsequently integrated into an information system.
Mandate
To advise the Minister of Health and Social Services on any risks associated with blood transfusions, infections that are spread through blood and any new relevant information on blood products.
Structure
The Biovigilance Committee is composed of three representatives of users, and experts in the fields of transfusion medicine, public health and ethics. Three people, among whom, a representative of the supplier (Héma-Québec) have a seat on the committee as observers.
Members
For the members list, visit the Biovigilance Committee site.
